Disposable medical masks are essential products in global healthcare and hygiene systems. However, requirements for medical masks vary significantly across different countries and regions. For manufacturers, importers, and distributors, choosing the right disposable medical mask for each target market is critical to ensuring regulatory compliance, smooth customs clearance, and successful long-term sales.
This guide explains how to select disposable medical masks for different international markets in 2026, with a focus on standards, performance requirements, and buyer expectations.
1. Why Market-Specific Selection Matters
A medical mask that is accepted in one country may not meet the regulatory requirements of another. Differences may include:
Certification standards
Testing methods
Labeling and packaging rules
Intended use classifications
Failing to meet local requirements can result in delayed shipments, rejected imports, or product recalls.
Understanding the regulatory framework of each target market is the first step in choosing the right product.
2. Key Global Medical Mask Standards Overview
While standards differ by region, most medical masks are evaluated based on:
Bacterial Filtration Efficiency (BFE)
Differential Pressure (breathability)
Splash or fluid resistance
Biocompatibility
Below is an overview of the most commonly recognized standards.
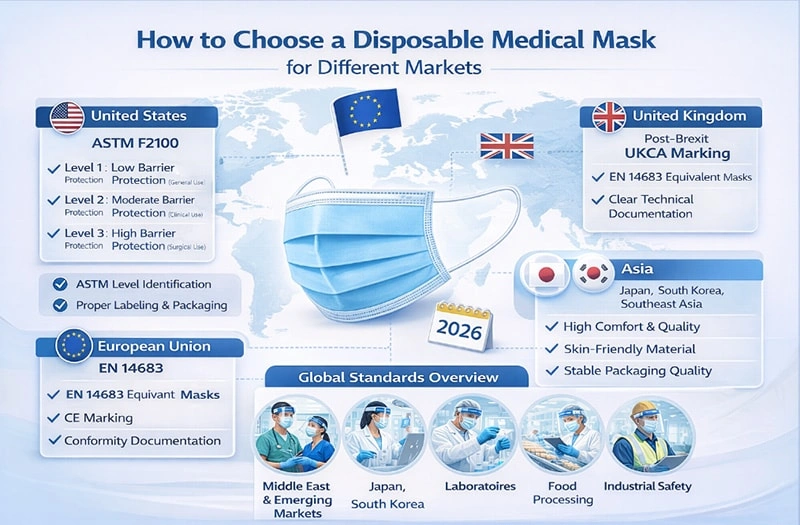
3. United States Market (ASTM F2100)
In the United States, disposable medical masks are regulated under ASTM F2100, which defines three performance levels:
Level 1: Low barrier protection (general medical use)
Level 2: Moderate barrier protection (clinical environments)
Level 3: High barrier protection (high fluid exposure procedures)
U.S. buyers typically prioritize:
Clear ASTM level identification
Consistent BFE performance
Proper labeling and packaging
Medical masks are widely used in hospitals, clinics, dental offices, and outpatient care facilities.
4. European Union Market (EN 14683)
In the European Union, disposable medical masks must comply with EN 14683, which includes:
Type I: Basic protection
Type II: Higher filtration efficiency
Type IIR: Type II with splash resistance
Type IIR masks are commonly used in medical and surgical environments due to their fluid resistance.
EU buyers often emphasize:
CE marking
Conformity documentation
Traceability and quality management systems
5. United Kingdom Market
Post-Brexit, the UK generally follows standards aligned with EN 14683. While regulatory procedures differ, product performance requirements remain similar.
UK importers typically expect:
Clear technical documentation
Compliance with recognized medical standards
Reliable supply consistency
6. Asian Markets (Japan, South Korea, Southeast Asia)
Asian markets vary widely in regulatory structure and buyer expectations.
Common trends include:
High focus on product comfort and breathability
Preference for lightweight, skin-friendly materials
Strong demand for stable quality and packaging consistency
In Japan and South Korea, buyers often require:
High-quality manufacturing processes
Clear quality documentation
Attention to user experience
7. Middle East and Emerging Markets
In many Middle Eastern, African, and Latin American markets, medical mask requirements may be less standardized but still focus on:
BFE performance
Basic safety and hygiene compliance
Competitive pricing with reliable quality
Government tenders and institutional buyers may specify:
Minimum filtration performance
Country-of-origin labeling
Bulk packaging requirements
8. Choosing Mask Specifications Based on Market Needs
When selecting a disposable medical mask for export, consider the following factors:
Filtration performance: Match BFE levels to local standards
Breathability: Balance protection and wearer comfort
Fluid resistance: Required in surgical or high-risk settings
Earloop or tie-on design: Depends on local preference
Packaging format: Individual, retail box, or bulk carton
Different markets may prioritize different combinations of these features.
9. Labeling, Packaging, and Documentation
Compliance is not limited to the mask itself. Packaging and documentation play a crucial role in market acceptance.
Important elements include:
Product name and classification
Applicable standard reference
Manufacturer and origin information
Lot number and traceability
Accurate and compliant labeling helps ensure smoother customs clearance and buyer confidence.
10. Building Trust with International Buyers
Beyond compliance, international buyers look for suppliers who demonstrate:
Consistent production quality
Transparent communication
Understanding of local market needs
Long-term supply stability
Providing clear technical information and market-specific guidance positions suppliers as professional partners rather than commodity sellers.
Conclusion
Choosing the right disposable medical mask for different markets requires more than selecting a product with basic filtration performance. It involves understanding regulatory standards, buyer expectations, and application environments across regions.
By aligning product specifications with market-specific requirements, manufacturers and distributors can ensure compliance, improve buyer confidence, and achieve sustainable growth in global medical mask markets.


 09-Feb--2026
09-Feb--2026